Evidence indicates that human milk oligosaccharides (HMOs) have different biological functions important for healthy development in early life.
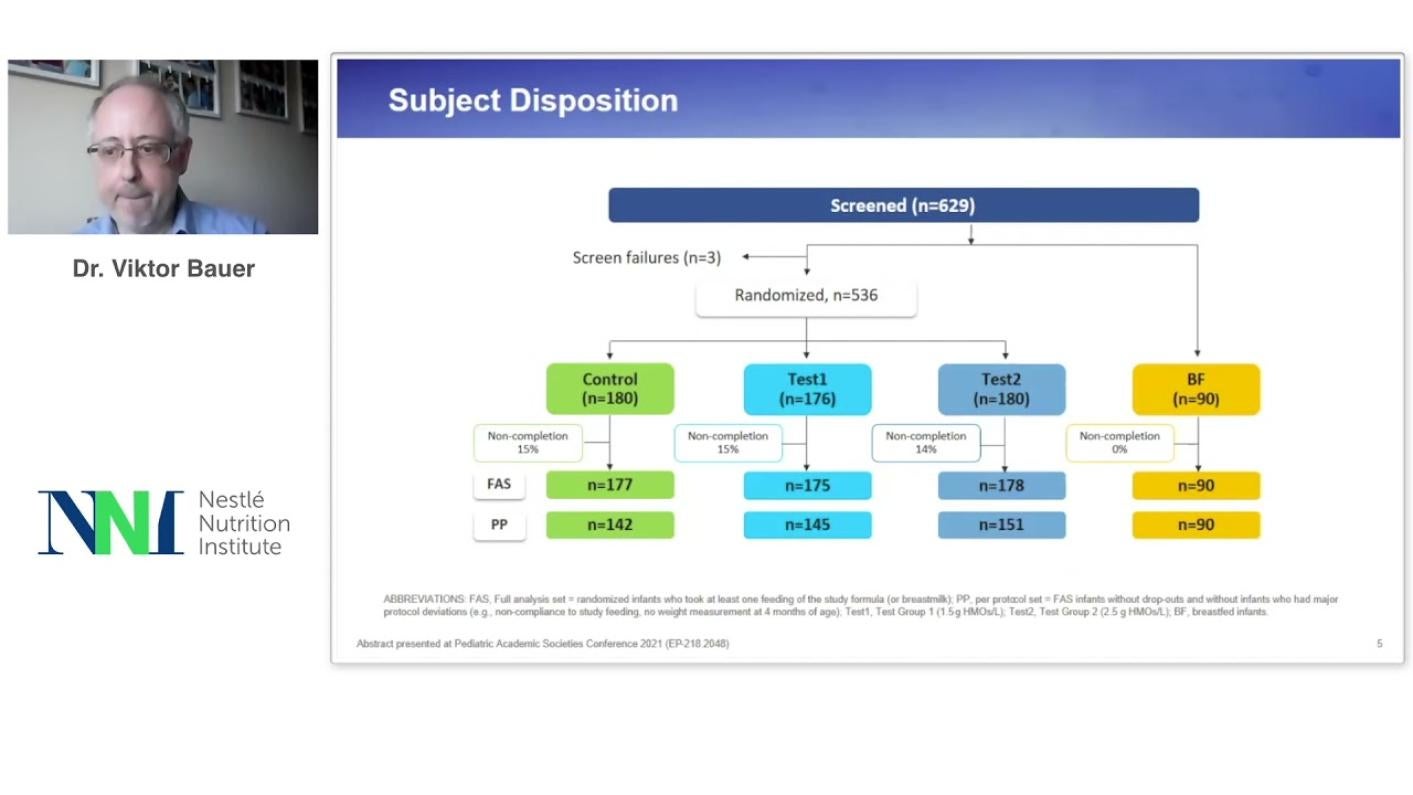
This trial evaluated growth, safety and tolerance in infants fed an infant formula supplemented with a blend of 5 HMOs structurally identical to those in breastmilk: 2’- fucosyllactose, 2',3-di-fucosyllactose, lacto-N-tetraose, 3’-sialyllactose, and 6’-sialyllactose. This HMO blend is designed to represent the major HMO classes and based on the HMO profile found in breastmilk.
This is the first clinical trial done with infant formula supplemented with a diverse blend of five HMOs and have shown as a result: age-appropriate growth and soft stooling pattern, as well as safe and adequate tolerance with the tested formula.